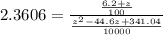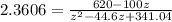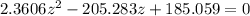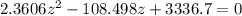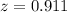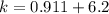Chemistry, 21.10.2020 16:01 ineemorehelp
While ethanol CH3CH2OH is produced naturally by fermentation, e. g. in beer- and wine-making, industrially it is synthesized by reacting ethylene CH2CH2 with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 100L tank with 33.mol of ethylene gas and 16.mol of water vapor. When the mixture has come to equilibrium he determines that it contains 26.8mol of ethylene gas and 9.8mol of water vapor. The engineer then adds another 8.0mol of water, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
While ethanol CH3CH2OH is produced naturally by fermentation, e. g. in beer- and wine-making, indust...
Questions

Mathematics, 25.07.2019 07:30

Mathematics, 25.07.2019 07:30


Mathematics, 25.07.2019 07:30


Mathematics, 25.07.2019 07:30


Biology, 25.07.2019 07:30

Social Studies, 25.07.2019 07:30



Social Studies, 25.07.2019 07:30


Business, 25.07.2019 07:30

Health, 25.07.2019 07:30

Health, 25.07.2019 07:30

Health, 25.07.2019 07:30



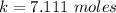
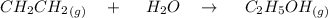
![K = \frac{[C_2H_5OH]}{[CH_2CH_2] [H_2O]}](/tpl/images/0827/7982/45f06.png)
![[C_2H_5OH]](/tpl/images/0827/7982/ff8e1.png) is the concentration of ethanol which is mathematically represented as
is the concentration of ethanol which is mathematically represented as ![[C_2H_5OH] = \frac{6.2 \ mol}{100L}](/tpl/images/0827/7982/5759e.png)
![[C_2H_5OH] = 0.062 mol/L](/tpl/images/0827/7982/bdf4f.png)
![[CH_2CH_2] = \frac{26.8 \ mol}{100L}](/tpl/images/0827/7982/29e7b.png)
![[CH_2CH_2] = 0.268 mol/L](/tpl/images/0827/7982/35c2d.png)
![[H_2O] = \frac{ 9.8 \ mol}{100L}](/tpl/images/0827/7982/6e32c.png)
![[H_2O] = 0.098 mol/L](/tpl/images/0827/7982/7f4e9.png)
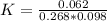
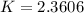
![[C_2H_5OH] = \frac{6.2+z}{100}](/tpl/images/0827/7982/6dfac.png)
![[CH_2CH_2]= \frac{ 34.8- z }{100}](/tpl/images/0827/7982/fc946.png)
![[CH_2CH_2]= \frac{ 9.8-z }{100}](/tpl/images/0827/7982/549ef.png)
![2.3606 = \frac{ \frac{6.2+z}{100}}{[ \frac{34.8- z }{100}] [\frac{ 9.8-z }{100}]}](/tpl/images/0827/7982/817fe.png)