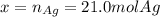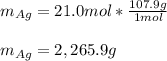Chemistry, 21.10.2020 16:01 thedaisylopez3628
Assume that silver and gold form ideal, random mixtures. Calculate the mass of pure Ag needed to cause an entropy increase of 20 J/K when mixed with 100g of pure Au

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Assume that silver and gold form ideal, random mixtures. Calculate the mass of pure Ag needed to cau...
Questions

Social Studies, 12.11.2020 20:10


History, 12.11.2020 20:10


Mathematics, 12.11.2020 20:10

Engineering, 12.11.2020 20:10

Mathematics, 12.11.2020 20:10

Mathematics, 12.11.2020 20:10

Mathematics, 12.11.2020 20:10


Biology, 12.11.2020 20:10



Business, 12.11.2020 20:10

Mathematics, 12.11.2020 20:10

History, 12.11.2020 20:10

Chemistry, 12.11.2020 20:10

Mathematics, 12.11.2020 20:10


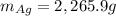
![\Delta S=-n_TR\Sigma[x_i*ln(x_i)]](/tpl/images/0827/7699/ef17c.png)
![\Delta S=-(n_{Au}+n_{Ag})R\Sigma[\frac{n_{Au}}{n_{Au}+n_{Ag}} *ln(\frac{n_{Au}}{n_{Au}+n_{Ag}} )+\frac{n_{Ag}}{n_{Au}+n_{Ag}} *ln(\frac{n_{Ag}}{n_{Au}+n_{Ag}} )]](/tpl/images/0827/7699/1af49.png)
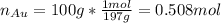
 representing the moles of silver:
representing the moles of silver:![20\frac{J}{mol}=-(0.508+x)8.314\frac{J}{mol*K} \Sigma[\frac{0.508}{0.508+x} *ln(\frac{0.508}{0.508+x} )+\frac{x}{0.508+x} *ln(\frac{x}{0.508+x} )]](/tpl/images/0827/7699/365c1.png)