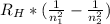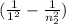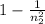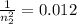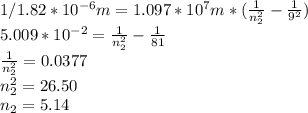Chemistry, 24.09.2019 17:30 kkelley9223
Aground state hydrogen atom absorbs a photon of light having a wavelength of 92.30 nm. it then gives off a photon having a wavelength of 1820 nm. what is the final state of the hydrogen atom?

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:02
If the empirical formula of a compound is known what is needed in order to determine the molecular formula a) the coordination numbers b)the molecular geometry c) the molar mass
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Aground state hydrogen atom absorbs a photon of light having a wavelength of 92.30 nm. it then gives...
Questions


Mathematics, 24.02.2021 07:30



Mathematics, 24.02.2021 07:30


Engineering, 24.02.2021 07:30

Chemistry, 24.02.2021 07:30



Chemistry, 24.02.2021 07:30

Mathematics, 24.02.2021 07:30



English, 24.02.2021 07:30


Chemistry, 24.02.2021 07:30

Mathematics, 24.02.2021 07:30

Mathematics, 24.02.2021 07:30

Chemistry, 24.02.2021 07:30