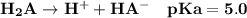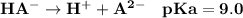Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
You make up a solution of a diprotic acid, H2A, having pKa values of 5.0 and 9.0. Identify the prima...
Questions


Mathematics, 08.03.2021 14:00

Biology, 08.03.2021 14:00

Geography, 08.03.2021 14:00


Biology, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00

Chemistry, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00


Social Studies, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00

Biology, 08.03.2021 14:00

Geography, 08.03.2021 14:00

Chemistry, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00

Computers and Technology, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00

Computers and Technology, 08.03.2021 14:00

English, 08.03.2021 14:00