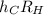Chemistry, 21.10.2020 04:01 logsdonella
With some manipulation, the Rydberg equation can be rewritten in the form
E=constant×(1nf2−1ni2)
which allows you to calculate the energy of the emitted light. Express this constant in terms of the constants h, c, and RH using relationships between wavelength and energy as well as the Rydberg equation from the introduction.
Express the constant in terms of h and c, and RH.
Please help me with this question?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1
You know the right answer?
With some manipulation, the Rydberg equation can be rewritten in the form
E=constant×(1nf2−1ni2)
Questions

English, 15.12.2019 22:31

Mathematics, 15.12.2019 22:31

History, 15.12.2019 22:31


Business, 15.12.2019 22:31

Mathematics, 15.12.2019 22:31

Mathematics, 15.12.2019 22:31




Mathematics, 15.12.2019 22:31

Mathematics, 15.12.2019 22:31


Biology, 15.12.2019 22:31


English, 15.12.2019 22:31

History, 15.12.2019 22:31

Biology, 15.12.2019 22:31


History, 15.12.2019 22:31