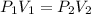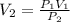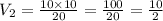Chemistry, 21.10.2020 03:01 PONBallfordM89
Given a constant temperature, if a gas has a volume of 10 L at a pressure of 10 atm, what would be the volume of the gas at 20 atm? Show all of your work.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Given a constant temperature, if a gas has a volume of 10 L at a pressure of 10 atm, what would be t...
Questions




Mathematics, 15.10.2020 20:01



Chemistry, 15.10.2020 20:01

Mathematics, 15.10.2020 20:01

Mathematics, 15.10.2020 20:01


English, 15.10.2020 20:01

Chemistry, 15.10.2020 20:01

Mathematics, 15.10.2020 20:01





Mathematics, 15.10.2020 20:01

Biology, 15.10.2020 20:01