Chemistry, 21.10.2020 03:01 lasvegas5811
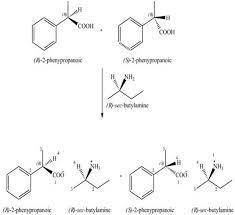
An acid-base reaction of (r)-sec-butylamine with a racemic mixture of 2-phenylpropanoic acid forms two products having different melting points and somewhat different solubilities. Draw the structure of these two products. How are the two products related? Be sure to answer all parts.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
An acid-base reaction of (r)-sec-butylamine with a racemic mixture of 2-phenylpropanoic acid forms t...
Questions


Mathematics, 19.07.2019 15:40


Physics, 19.07.2019 15:40

Mathematics, 19.07.2019 15:40



Mathematics, 19.07.2019 15:40



Chemistry, 19.07.2019 15:40


Computers and Technology, 19.07.2019 15:40



Computers and Technology, 19.07.2019 15:40




English, 19.07.2019 15:40