
Chemistry, 20.10.2020 16:01 Flameking1223
Aspirin is a weak organic acid whose molecular formula is HC9H7O4. An aqueous solution of aspirin is prepared by dissolving 3.60 g/L. The pH of this solution is found to be 2.6. Calculate Ka for aspirin. (atomic mass: C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Aspirin is a weak organic acid whose molecular formula is HC9H7O4. An aqueous solution of aspirin is...
Questions

Physics, 16.02.2021 14:00

Social Studies, 16.02.2021 14:00

English, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

English, 16.02.2021 14:00

English, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Physics, 16.02.2021 14:00

Chemistry, 16.02.2021 14:00




English, 16.02.2021 14:00



Mathematics, 16.02.2021 14:00

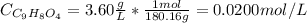
![Ka = \frac{[C_{9}H_{7}O_{4}^{-}][H_{3}O^{+}]}{[C_{9}H_{8}O_{4}]}](/tpl/images/0824/0933/a1a8e.png)
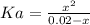
![pH = -log[H_{3}O^{+}]](/tpl/images/0824/0933/b7638.png)
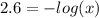
![x = 2.51 \cdot 10^{-3} M = [H_{3}O^{+}] = [C_{9}H_{7}O_{4}^{-}]](/tpl/images/0824/0933/98904.png)
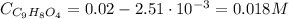
![Ka = \frac{[C_{9}H_{7}O_{4}^{-}][H_{3}O^{+}]}{[C_{9}H_{8}O_{4}]} = \frac{(2.51 \cdot 10^{-3})^{2}}{0.018} = 3.50 \cdot 10^{-4}](/tpl/images/0824/0933/393ec.png)


