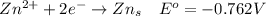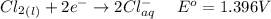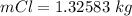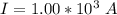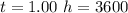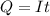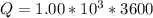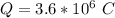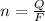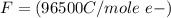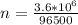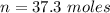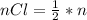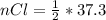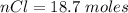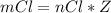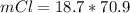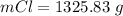Consider the rechargeable battery: Zn(s)0ZnCl (aq)7Cl2(aq)0Cl (l)0C(s) (a) Write reduction half-reactions for each electrode. From which electrode will electrons flow from the battery into a circuit if the electrode potentials are not too different from E 8 values

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Consider the rechargeable battery: Zn(s)0ZnCl (aq)7Cl2(aq)0Cl (l)0C(s) (a) Write reduction half-reac...
Questions

Computers and Technology, 08.06.2021 19:00

Mathematics, 08.06.2021 19:00

Social Studies, 08.06.2021 19:00




Mathematics, 08.06.2021 19:00




Mathematics, 08.06.2021 19:00

Geography, 08.06.2021 19:00


Mathematics, 08.06.2021 19:00


Mathematics, 08.06.2021 19:00

Business, 08.06.2021 19:00



Mathematics, 08.06.2021 19:00

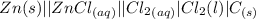
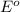 values
values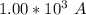 for 1.00 h , how many kg of
for 1.00 h , how many kg of 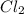 will be consumed
will be consumed