
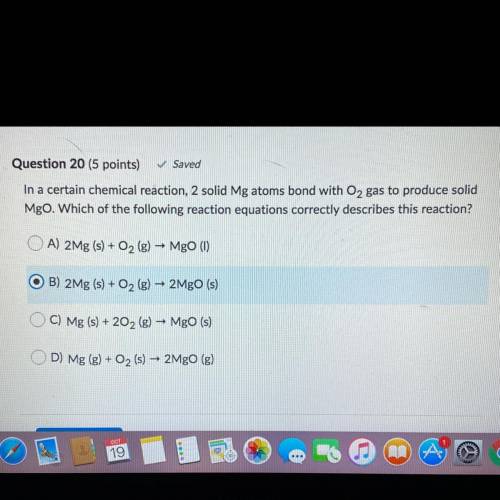
In a certain chemical reaction, 2 solid Mg atoms bond with O2 gas to produce solid
Mgo. Which of the following reaction equations correctly describes this reaction?
A) 2Mg (s) + O2(g) – Mgo (1)
O B) 2Mg (s) + O2 (g) – 2Mgo (s)
Sey
C) Mg (s) + 202 (g) – Mgo (s)
D) Mg (g) + O2 (s)
-
2MgO (g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 11:30
If 4.8 moles of x and 3.4 moles of y react according to the reaction below, how many moles of the excess reactant will be left over at the end of the reaction? 3x + 2y “yields”/ x3y2. a. 1.7 mol y left over b. 1.6 mol x left over c. 0.2 mol y left over d. 0.1 mol x left over
Answers: 1
You know the right answer?
In a certain chemical reaction, 2 solid Mg atoms bond with O2 gas to produce solid
Mgo. Which of th...
Questions

Mathematics, 25.07.2019 04:40







Biology, 25.07.2019 04:40


English, 25.07.2019 04:40



History, 25.07.2019 04:40



Mathematics, 25.07.2019 04:40

Mathematics, 25.07.2019 04:40



History, 25.07.2019 04:40




