Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
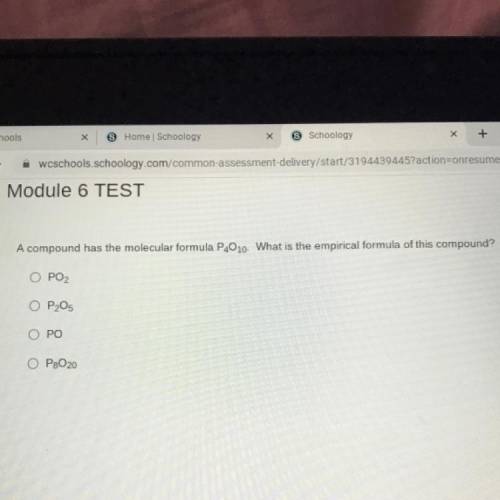
A compound has the molecular formula P4O10 What is the empirical formula of this compound?
PO2
Questions

Chemistry, 19.04.2021 18:20

Mathematics, 19.04.2021 18:20

English, 19.04.2021 18:20

English, 19.04.2021 18:20


Mathematics, 19.04.2021 18:20


Mathematics, 19.04.2021 18:20

Mathematics, 19.04.2021 18:20



Mathematics, 19.04.2021 18:20



Social Studies, 19.04.2021 18:20




Mathematics, 19.04.2021 18:20