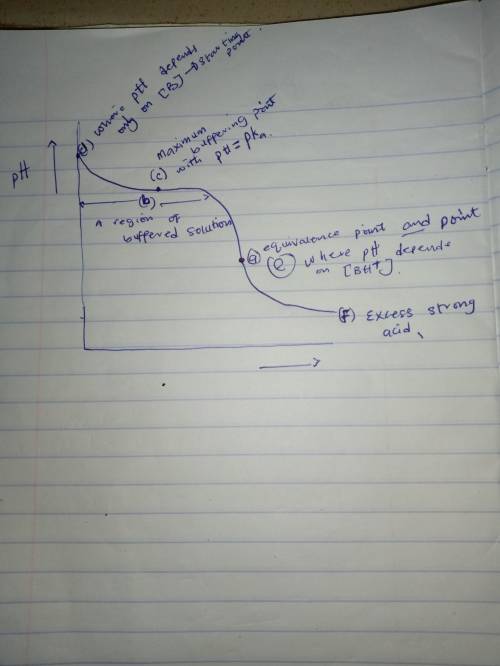
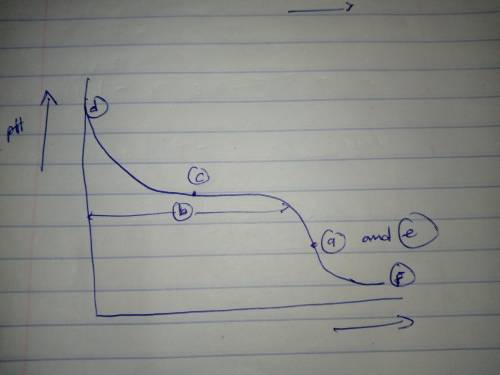
Sketch the titration curve for the titration of a generic weak base B with a strong acid. The titration reaction is
B+H+⇌BH+
On the curve indicate the points that correspond to the following:
a) the equivalence point.
b) the region with maximum buffering) the point where pH = pKbd) the region where pH depends only on [B]e) the region where pH depends only on [BH+]f) the region where pH depends only on the amount of excess strong acid

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Sketch the titration curve for the titration of a generic weak base B with a strong acid. The titrat...
Questions


Arts, 20.01.2021 19:20



English, 20.01.2021 19:20



Mathematics, 20.01.2021 19:20



Mathematics, 20.01.2021 19:20

Mathematics, 20.01.2021 19:20

Chemistry, 20.01.2021 19:20


Mathematics, 20.01.2021 19:20

Biology, 20.01.2021 19:20

Arts, 20.01.2021 19:20