Chemistry, 19.10.2020 14:01 samyajones68
Using the reaction below: 2 CO2(g) + 2 H2O(l) → C2H4(g) + 3 O2(g) ΔHrxn= +1411.1 kJ What would be the heat of reaction for this reaction? 0.5 C2H4(g) + 1.5 O2(g) → CO2(g) + H2O(l) ΔHrxn= ??? KJ Question 6 options: a) Not enough information is given b) -2822.2 kJ c) +1411.1 kJ d) -705.55 kJ e) -1411.1 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1


Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2
You know the right answer?
Using the reaction below: 2 CO2(g) + 2 H2O(l) → C2H4(g) + 3 O2(g) ΔHrxn= +1411.1 kJ What would be th...
Questions



English, 10.12.2020 20:20







Biology, 10.12.2020 20:20



Mathematics, 10.12.2020 20:20



Biology, 10.12.2020 20:20


Medicine, 10.12.2020 20:20


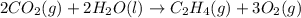



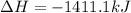
 , enthalpy gets half:
, enthalpy gets half: