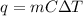Chemistry, 18.10.2020 15:01 bikerhomie
Problem:
Determine the amount of energy (heat) in Joules and Kcal required to raise the temperature 0f 98.5 grams water from 37.0 0C to 75.0 0C.
Restate the problem (in your own words & all relevant information included).
Model that best represents the problem. Draw and label.
Steps to solve the problem (include formula, known and unknown variables, etc)
Solution and final answer. (show cancellation of units).
Reflection: Must at least have 3 sentences.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

You know the right answer?
Problem:
Determine the amount of energy (heat) in Joules and Kcal required to raise the temperature...
Questions

Mathematics, 28.01.2021 04:40

History, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40

English, 28.01.2021 04:40


Mathematics, 28.01.2021 04:40


Mathematics, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40


Mathematics, 28.01.2021 04:40




Mathematics, 28.01.2021 04:40


Geography, 28.01.2021 04:40

Mathematics, 28.01.2021 04:40