
Chemistry, 18.10.2020 05:01 cshopholic4921
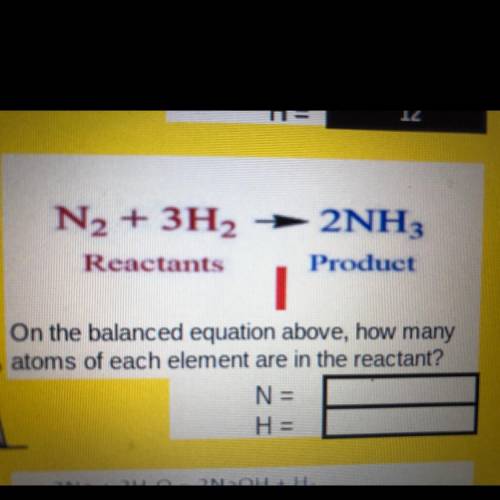
2NH.
N2 + 3H2
Reactants
Product
On the balanced equation above, how many
atoms of each element are in the reactant?
N =
H =

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1


Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
2NH.
N2 + 3H2
Reactants
Product
On the balanced equation above, how many
at...
Reactants
Product
On the balanced equation above, how many
at...
Questions




Spanish, 23.08.2019 10:50


Social Studies, 23.08.2019 10:50

Social Studies, 23.08.2019 10:50

Mathematics, 23.08.2019 10:50







Mathematics, 23.08.2019 10:50


Geography, 23.08.2019 10:50


Biology, 23.08.2019 10:50

Mathematics, 23.08.2019 10:50



