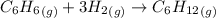The initial pressure of a mixture of C6H6 and an excess of H2 in a rigid vessel is 1.21 atm. A catalyst is introduced. After the reaction reaches completion, the temperature is restored to its initial value. The final pressure in the vessel is 0.839 atm. What was the mole fraction of C6H6 in the original mixture

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
The initial pressure of a mixture of C6H6 and an excess of H2 in a rigid vessel is 1.21 atm. A catal...
Questions

Social Studies, 03.02.2020 20:52



Biology, 03.02.2020 20:52


History, 03.02.2020 20:52






English, 03.02.2020 20:52


Mathematics, 03.02.2020 20:52


Chemistry, 03.02.2020 20:52


Physics, 03.02.2020 20:52

Mathematics, 03.02.2020 20:52