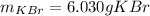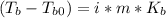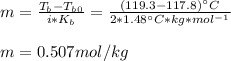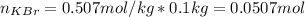Chemistry, 17.10.2020 14:01 sconner733
A certain liquid has a normal boiling point of and a boiling point elevation constant . A solution is prepared by dissolving some potassium bromide () in of . This solution boils at . Calculate the mass of that was dissolved.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1


Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
A certain liquid has a normal boiling point of and a boiling point elevation constant . A solution i...
Questions



Physics, 26.01.2021 16:30

Mathematics, 26.01.2021 16:30


Mathematics, 26.01.2021 16:30

Social Studies, 26.01.2021 16:30

Spanish, 26.01.2021 16:30

English, 26.01.2021 16:30

Mathematics, 26.01.2021 16:30

Biology, 26.01.2021 16:30



Health, 26.01.2021 16:30

Advanced Placement (AP), 26.01.2021 16:30





Mathematics, 26.01.2021 16:30