Chemistry, 16.10.2020 14:01 abelxoconda
State Hess' law of constant heat summation.
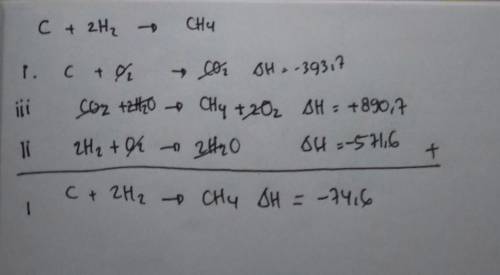
(b) Calculate the enthalpy of formation of CH4 from the following data:
i) C(s) + O2(g) → CO2(g); ∆H = -393.7 kJ/mol
ii) H2(g) + 1⁄2 O2(g) → H2O(l); ∆H = -285.8 kJ/mol
iii) CH4(g) + 2 O2(g)→ CO2(g) + 2H2O(l); ∆H = -890.4 kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2
You know the right answer?
State Hess' law of constant heat summation.
(b) Calculate the enthalpy of formation of CH4 from the...
Questions

History, 02.04.2020 23:35





Mathematics, 02.04.2020 23:35






Health, 02.04.2020 23:35


Biology, 02.04.2020 23:35

English, 02.04.2020 23:35


Chemistry, 02.04.2020 23:35

Biology, 02.04.2020 23:35

History, 02.04.2020 23:35