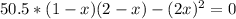Chemistry, 16.10.2020 14:01 williamsjako
A 1.00-L flask is filled with 1.00 moles of H2 and 2.00 moles of I2. The value of the equilibrium constant for the reaction of hydrogen and iodine reacting to form hydrogen iodide is 50.5 under the given conditions. What are the equilibrium concentrations of H2 , I2 , and HI in moles/L? H2 (g) + I2 (g) ⇌ 2HI(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
You know the right answer?
A 1.00-L flask is filled with 1.00 moles of H2 and 2.00 moles of I2. The value of the equilibrium co...
Questions

History, 27.12.2019 21:31

Biology, 27.12.2019 21:31


Mathematics, 27.12.2019 21:31

Mathematics, 27.12.2019 21:31

History, 27.12.2019 21:31



Mathematics, 27.12.2019 21:31






Social Studies, 27.12.2019 21:31


Mathematics, 27.12.2019 21:31


Social Studies, 27.12.2019 21:31

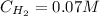
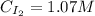
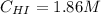
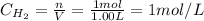
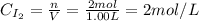
![Kc = \frac{[HI]^{2}}{[H_{2}][I_{2}]} = \frac{(2x)^{2}}{(1-x)(2-x)}](/tpl/images/0811/0470/b43fa.png)