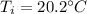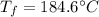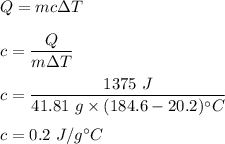Chemistry, 16.10.2020 15:01 tegaoks6843
A 41.81 g sample of a substance is initially at 20.2 °C. After absorbing 1375 J of heat, the temperature of the substance is 184.6 °C. What is the specific heat (c) of the substance?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2
You know the right answer?
A 41.81 g sample of a substance is initially at 20.2 °C. After absorbing 1375 J of heat, the tempera...
Questions

History, 23.01.2020 11:31

Mathematics, 23.01.2020 11:31





History, 23.01.2020 11:31

History, 23.01.2020 11:31

Business, 23.01.2020 11:31



Mathematics, 23.01.2020 11:31

Computers and Technology, 23.01.2020 11:31


Mathematics, 23.01.2020 11:31



Physics, 23.01.2020 11:31


Advanced Placement (AP), 23.01.2020 11:31