
Chemistry, 16.10.2020 08:01 zariahirons44
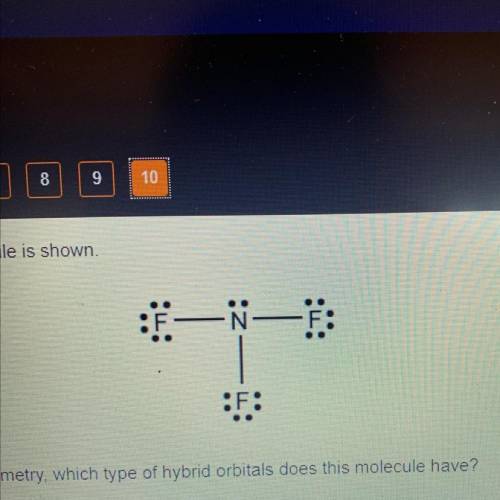
A Lewis electron structure of a molecule is shown.
Judging by the electron domain geometry, which type of hybrid orbitals does this molecule have?
sp
sp?
N
sp3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

You know the right answer?
A Lewis electron structure of a molecule is shown.
Judging by the electron domain geometry, which t...
Questions


Mathematics, 18.08.2020 14:01



Mathematics, 18.08.2020 14:01


Mathematics, 18.08.2020 14:01

Mathematics, 18.08.2020 14:01

Computers and Technology, 18.08.2020 14:01

Mathematics, 18.08.2020 14:01

Mathematics, 18.08.2020 14:01



Biology, 18.08.2020 14:01





Health, 18.08.2020 14:01

History, 18.08.2020 14:01



