Chemistry, 16.10.2020 07:01 briannabo08
Two chemicals A and B are combined to form a chemical C. The rate, or velocity, of the reaction is proportional to the product of the instantaneous amounts of A and B not converted to chemical C. Initially, there are 100 grams of A and 50 grams of B, and for each gram of B, 2 grams of A is used. It is observed that 10 grams of C is formed in 7 minutes. How much is formed in 28 minutes? (Round your answer to one decimal place.) grams What is the limiting amount of C after a long time? grams How much of chemicals A and B remains after a long time? A grams B grams At what time is chemical C half-formed? t = min

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Two chemicals A and B are combined to form a chemical C. The rate, or velocity, of the reaction is p...
Questions

English, 16.07.2019 14:10







English, 16.07.2019 14:10






Mathematics, 16.07.2019 14:10


History, 16.07.2019 14:10


History, 16.07.2019 14:10

Spanish, 16.07.2019 14:10

Social Studies, 16.07.2019 14:10

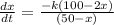
![\frac{1}{[(100 - 2x)(50 - x)]} dx = -k dt\\\\\frac{1}{2[(50 - x)(50 - x)]} dx = -k dt\\\\\ integral\ \frac{1}{2[(50 - x)^2]} dx =\ integral [-k ] \ dt\\\\\frac{-1}{[100-2x]} = -kt + D \\\\](/tpl/images/0809/8394/56d81.png)
![\frac{-1}{[100-2x]} = -kt + D \\\\\frac{ -1}{[100]} = 0 + D\\\\D= \frac{-1}{100}\\\\](/tpl/images/0809/8394/9d504.png)
![\frac{-1}{[100-2x]}= -kt -\frac{1}{100}\\\\or \\\\ \frac{1}{(100-2x)} = kt + \frac{1}{100}](/tpl/images/0809/8394/b58aa.png)
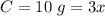
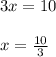
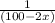 equation
equation 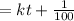 to get k.
to get k. 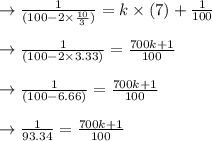

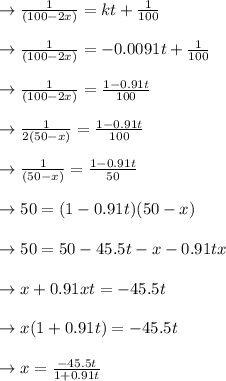
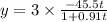
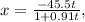 plug t = 28
plug t = 28