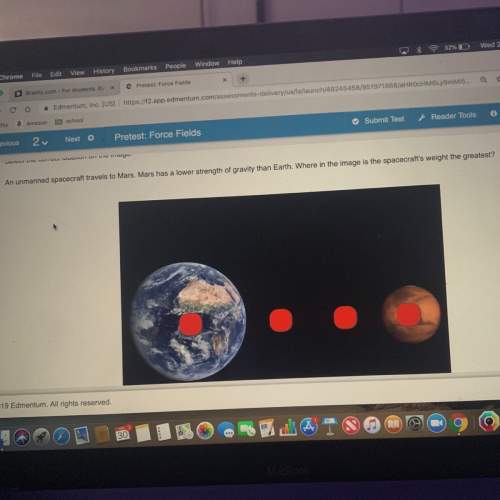
Chemistry, 16.10.2020 06:01 lupitaca888
The first step in the metabolism of ethanol is dehydrogenation by reaction with nicotinamide-adenine dinucleotide (NAD): C2H5OH+NAD+→C2H4O+NADHWhat is the heat effect of this reaction upon metabolizing 10 g of ethanol from a typical cocktail? What is the total heat effect for complete metabolism of the 10 g of ethanol to CO2and water? How, if at all, is the perception of warmth that accompanies ethanol consumption related to these heat effects? For computing heat effects, you may neglect the temperature, pH, and ionic strength dependence of the enthalpy of reaction

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Given 7.65 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield?
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

You know the right answer?
The first step in the metabolism of ethanol is dehydrogenation by reaction with nicotinamide-adenine...
Questions

Mathematics, 07.11.2019 21:31

Social Studies, 07.11.2019 21:31




Biology, 07.11.2019 21:31




Mathematics, 07.11.2019 21:31

English, 07.11.2019 21:31






History, 07.11.2019 21:31

Chemistry, 07.11.2019 21:31

Mathematics, 07.11.2019 21:31

Mathematics, 07.11.2019 21:31