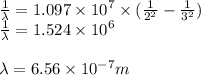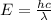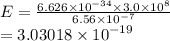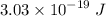Chemistry, 15.10.2020 04:01 itsyaboiamo
For a standard hydrogen atom, how much energy would be released for an electron in the third energy level to fall to the second energy level? Please show all calculations necessary.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2


Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
For a standard hydrogen atom, how much energy would be released for an electron in the third energy...
Questions

Mathematics, 31.03.2020 00:53



History, 31.03.2020 00:53

Mathematics, 31.03.2020 00:53

Biology, 31.03.2020 00:53

Mathematics, 31.03.2020 00:53

History, 31.03.2020 00:53

Physics, 31.03.2020 00:53


Mathematics, 31.03.2020 00:53

Mathematics, 31.03.2020 00:53







Mathematics, 31.03.2020 00:53

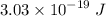
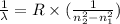
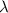 is the wavelength of the emitted photon
is the wavelength of the emitted photon