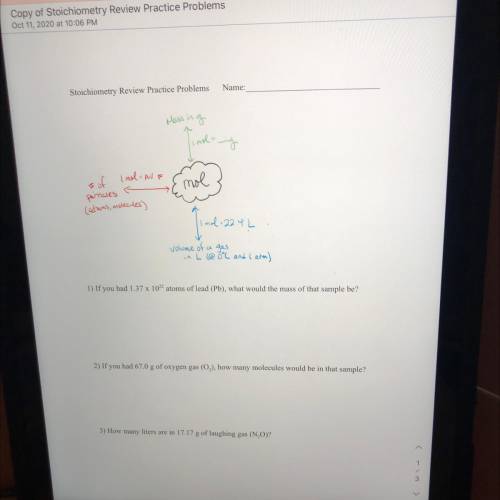
If you had 1.37 x 1022 atoms of lead (Pb), what would the mass of that sample be?
...

Chemistry, 15.10.2020 04:01 corbinfisher
If you had 1.37 x 1022 atoms of lead (Pb), what would the mass of that sample be?

Answers: 1


Another question on Chemistry




You know the right answer?
Questions

Mathematics, 11.02.2021 07:00

Mathematics, 11.02.2021 07:00


Mathematics, 11.02.2021 07:00


History, 11.02.2021 07:00


Biology, 11.02.2021 07:00

Mathematics, 11.02.2021 07:00

Arts, 11.02.2021 07:00

English, 11.02.2021 07:00






English, 11.02.2021 07:00

Chemistry, 11.02.2021 07:00


English, 11.02.2021 07:00



