How do I find the moles of OH- which reacted (mol) in the titration. Table Attached
...

Chemistry, 15.10.2020 04:01 Billyr9088
How do I find the moles of OH- which reacted (mol) in the titration. Table Attached
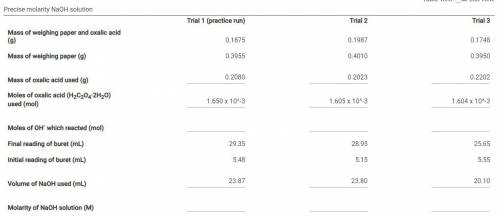

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
Questions


Mathematics, 09.02.2021 17:50


English, 09.02.2021 17:50

Mathematics, 09.02.2021 17:50



English, 09.02.2021 17:50




Arts, 09.02.2021 17:50




Mathematics, 09.02.2021 17:50



Mathematics, 09.02.2021 17:50



