
Chemistry, 15.10.2020 02:01 sydneyharding36191
The mass of a block is 12 g and the volume is 6 ml. Calculate the density of the block.
Question 2 options:
18 g/ml
72 g/ml
0.5 g/ml
2 g/ml

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3



Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
The mass of a block is 12 g and the volume is 6 ml. Calculate the density of the block.
Question 2...
Questions

Spanish, 19.10.2019 08:00

Health, 19.10.2019 08:00

History, 19.10.2019 08:00


Mathematics, 19.10.2019 08:00

Biology, 19.10.2019 08:00

History, 19.10.2019 08:00

Chemistry, 19.10.2019 08:00








History, 19.10.2019 08:00



History, 19.10.2019 08:00

Geography, 19.10.2019 08:00

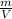
 = 2g/ml
= 2g/ml

