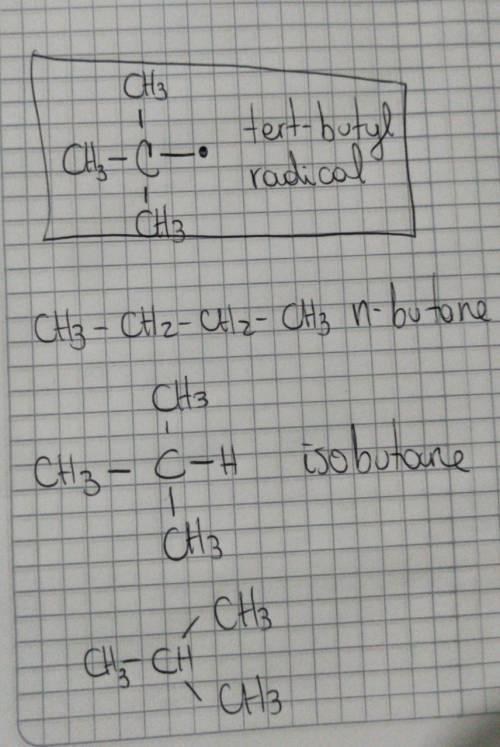
The molecular formula of butane is C4H10. It is obtained from petroleum and is used commonly in LPG (Liquefied Petroleum Gas) cylinders (a common source of cooking gas). It has two arrangements of carbon atoms: a straight chain and a branched chain. Using this information, draw the structure of the tertiary butyl radical that will form upon removal of a hydrogen atom. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
The molecular formula of butane is C4H10. It is obtained from petroleum and is used commonly in LPG...
Questions

Mathematics, 29.04.2021 19:10



Mathematics, 29.04.2021 19:10

Business, 29.04.2021 19:10

Mathematics, 29.04.2021 19:10

English, 29.04.2021 19:10

Mathematics, 29.04.2021 19:10

Advanced Placement (AP), 29.04.2021 19:10



History, 29.04.2021 19:10



Mathematics, 29.04.2021 19:10

Mathematics, 29.04.2021 19:10




Physics, 29.04.2021 19:10