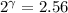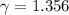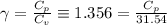The constant volume heat capacity of a gas can be measured by observing the decrease in temperature when it expands adiabatically and reversibly. If the decrease in pressure is also measured, we can use it to infer the value of γ = Cp/Cv and hence, by combining the two values, deduce the constant-pressure heat capacity. A fluorocarbon gas was allowed to expand reversibly and adiabatically to twice its volume; as a result, the temperature fell from 298.15 K to 248.44 K and its pressure fell from 202.94 kPa to 81.840 kPa. Evaluate Cp

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
The constant volume heat capacity of a gas can be measured by observing the decrease in temperature...
Questions





World Languages, 06.10.2020 01:01

Spanish, 06.10.2020 01:01


Mathematics, 06.10.2020 01:01


Mathematics, 06.10.2020 01:01





Biology, 06.10.2020 01:01



Mathematics, 06.10.2020 01:01


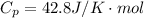
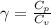
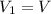

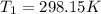
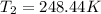
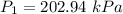
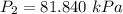
![T_2 = T_1 * [ \frac{V_1}{V_2} ]^{\frac{R}{C_v} }](/tpl/images/0802/4357/d7f78.png)
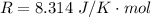
![248.44 = 298.15 * [ \frac{V}{2V} ]^{\frac{8.314}{C_v} }](/tpl/images/0802/4357/a72d7.png)
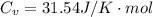
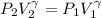
![[ 81.840 *10^3] [2V]^{\gamma} = [202.94 *10^3] V^{\gamma}](/tpl/images/0802/4357/43b7e.png)