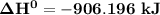Chemistry, 13.10.2020 03:01 giraffesaur44
The first reaction in the Ostwald process for the production of nitric acid involves the combustion of ammonia
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)
a) Estimate ΔH^o (in kJ) for this reaction using average bond energies.
b) Calculate ΔH^o (in kJ) for this reaction using standard heats of formation.
c) Briefly explain why the value for ΔH^o, calculated using average bond energies, is only considered to be an estimate of the standard enthalpy change for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
The first reaction in the Ostwald process for the production of nitric acid involves the combustion...
Questions


English, 02.03.2021 21:50

Engineering, 02.03.2021 21:50


Mathematics, 02.03.2021 21:50





Health, 02.03.2021 21:50

Mathematics, 02.03.2021 21:50

Chemistry, 02.03.2021 21:50

Mathematics, 02.03.2021 21:50

Mathematics, 02.03.2021 21:50

Mathematics, 02.03.2021 21:50


Mathematics, 02.03.2021 21:50

Social Studies, 02.03.2021 21:50

Social Studies, 02.03.2021 21:50

Social Studies, 02.03.2021 21:50

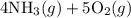 ↔
↔ 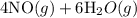
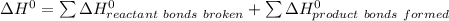
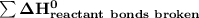 = 7182 kJ
= 7182 kJ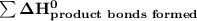 = - 8032 kJ
= - 8032 kJ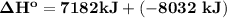

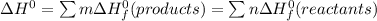
![\Delta H^0 = [ 4 \ mol \times \Delta H^0_f \ (NO(g)) + 6\ mol \times \Delta H^0_f(H_2O)] - [ 4 \ mol \times \Delta H^0_f \ (NH_3(g)) + 5 \ mol \times \Delta H^0_f \ (O_2)]](/tpl/images/0802/3228/2e247.png)
![\Delta H^0 = [ 4 \ mol \times90.29 \ kJ/mol + 6\ mol \times -241.826 \ kJ/mol - [ 4 \ mol \times-45.9 \ kJ/mol + 5 \ mol \times 0]](/tpl/images/0802/3228/35715.png)