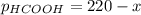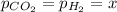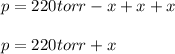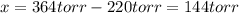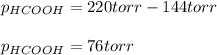Chemistry, 13.10.2020 03:01 91miketaylor
When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay: HCOOH(g) →CO2(g) + H2 (g) The rate of reaction is monitored by measuring the total pressure in the reaction container. Time (s) . . . P (torr) 0 . . . . . . . . . 220 50 . . . . . . . . 324 100 . . . . . . . 379 150 . . . . . . . 408 200 . . . . . . . 423 250 . . . . . . . 431 300 . . . . . . . 435 At the start of the reaction (time = 0), only formic acid is present. What is the formic acid pressure (in torr) when the total pressure is 364? Hint: use Dalton's law of partial pressure and the reaction stoichiometry.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay: HCO...
Questions



Chemistry, 18.03.2021 01:10





Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


Physics, 18.03.2021 01:10



History, 18.03.2021 01:10

English, 18.03.2021 01:10


Physics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

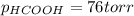
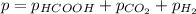
 as the time goes by:
as the time goes by: