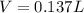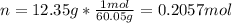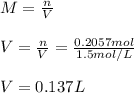Chemistry, 13.10.2020 01:01 onlymyworld27
It takes 12.35 grams of acetic acid (CH3COOH; molar mass of 60.05 g/mol) to prepare a 1.5 M solution. What is the volume in L?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1
You know the right answer?
It takes 12.35 grams of acetic acid (CH3COOH; molar mass of 60.05 g/mol) to prepare a
1.5 M solutio...
Questions

Mathematics, 18.03.2021 16:10





History, 18.03.2021 16:10

Mathematics, 18.03.2021 16:10

Mathematics, 18.03.2021 16:10



English, 18.03.2021 16:10

Mathematics, 18.03.2021 16:10

Chemistry, 18.03.2021 16:10





Computers and Technology, 18.03.2021 16:10

Mathematics, 18.03.2021 16:10