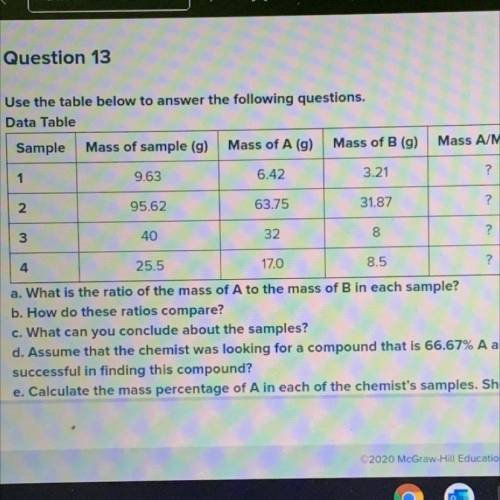
Use the table below to answer the following questions.
Data Table
Sample
Mass of sample...

Chemistry, 12.10.2020 05:01 marisolrojo2002
Use the table below to answer the following questions.
Data Table
Sample
Mass of sample (g) Mass of A (g) Mass of B (g)
1
9.63
6.42
3.21
Mass A/Mass B
?
2
95.62
63.75
31.87
?
3
40
32
8
?
4
25.5
170
8.5
?
a. What is the ratio of the mass of A to the mass of B in each sample?
b. How do these ratios compare?
c. What can you conclude about the samples?
d. Assume that the chemist was looking for a compound that is 66.67% A and 33.33% B. How could the chemist determine
successful in finding this compound?
w Our calculations

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
Questions



History, 06.04.2020 23:15







Mathematics, 06.04.2020 23:15

English, 06.04.2020 23:16



Mathematics, 06.04.2020 23:16

Mathematics, 06.04.2020 23:16







