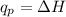Identify each enthalpy change by name and classify each change as exothermic or endothermic.
1. 1 mol nacl (s) à 1 mol nacl(l)
2. c4h10 o(l) à c4h10o (g)
3. h2o(g) à h2o (l)
4. 1 mol nacl(s) + 3.88 kj/molà 1 mol nacl(aq)
a molar heat of solution; endothermic
b molar heat of vaporization; endothermic
c molar heat of fusion; endothermic
d molar heat of condensation; exothermic

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
Identify each enthalpy change by name and classify each change as exothermic or endothermic.
Questions


Mathematics, 24.03.2021 16:40


Mathematics, 24.03.2021 16:40

Biology, 24.03.2021 16:40

Mathematics, 24.03.2021 16:40







Computers and Technology, 24.03.2021 16:40