
Chemistry, 11.10.2020 14:01 ohernandez35
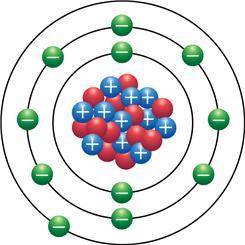
In the Bohr model of an atom (shown), protons are represented in blue, the neutrons are red and the electrons are green. How reactive is the element that this model represents?
the answer choices :
a. Highly reactive because the model has two complete electron shells
b. Highly reactive because the model is showing only one valence electron.
c. Non-reactive because the model is showing only one valence electron
d. Non-reactive because the model is showing two complete electron shells

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

You know the right answer?
In the Bohr model of an atom (shown), protons are represented in blue, the neutrons are red and the...
Questions

Social Studies, 05.10.2019 04:30



Social Studies, 05.10.2019 04:30


Mathematics, 05.10.2019 04:30

Business, 05.10.2019 04:30







Mathematics, 05.10.2019 04:30

Social Studies, 05.10.2019 04:30

Physics, 05.10.2019 04:30

History, 05.10.2019 04:30



Social Studies, 05.10.2019 04:30



