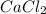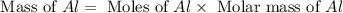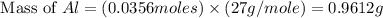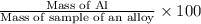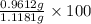5. A 1.1181-g sample of an alloy (a mixture) of aluminum and magnesium was treated with an
excess of sodium hydroxide solution. In the reaction, only the aluminum reacts with the sodium
hydroxide solution:
2 Al + 2 NaOH + 6 H202 Na[Al(OH)4] + 3 H2
If 0.1068 g of H2 is produced, what is the mass percent of aluminum in the alloy?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
5. A 1.1181-g sample of an alloy (a mixture) of aluminum and magnesium was treated with an
excess o...
Questions

Mathematics, 15.05.2021 21:30

English, 15.05.2021 21:30

Business, 15.05.2021 21:30

History, 15.05.2021 21:40


Mathematics, 15.05.2021 21:40



Mathematics, 15.05.2021 21:40


Mathematics, 15.05.2021 21:40

Spanish, 15.05.2021 21:40

History, 15.05.2021 21:40

Mathematics, 15.05.2021 21:40


Mathematics, 15.05.2021 21:40



Computers and Technology, 15.05.2021 21:40

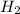 = 0.1068 g
= 0.1068 g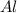 = 27 g/mol
= 27 g/mol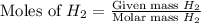
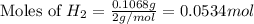
![2Al+2NaOH+6H_2O\rightarrow 2Na[Al(OH)_4]+3H_2](/tpl/images/0796/4861/bae6d.png)
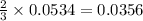 mole of
mole of