
Isoamyl salicylate ( = 208.25 g/mol) has a pleasant aroma and is used in perfumes and soaps. which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/ml)?
a. 117.2 g isoamyl salicylate in 950.0 ml of ethyl alcohol
b. 117.2 g isoamyl salicylate in 750.0 ml of ethyl alcohol
c. 117.2 g isoamyl salicylate in 750.0 ml of solution
d. 117.2 g isoamyl salicylate in 592.0 g of ethyl alcohol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1
You know the right answer?
Isoamyl salicylate ( = 208.25 g/mol) has a pleasant aroma and is used in perfumes and soaps. which o...
Questions


Mathematics, 11.03.2021 09:10


Mathematics, 11.03.2021 09:10

Mathematics, 11.03.2021 09:10



Biology, 11.03.2021 09:10

Mathematics, 11.03.2021 09:10

Mathematics, 11.03.2021 09:10

History, 11.03.2021 09:10


Mathematics, 11.03.2021 09:10

English, 11.03.2021 09:10


Mathematics, 11.03.2021 09:10

Mathematics, 11.03.2021 09:10

Mathematics, 11.03.2021 09:20

Biology, 11.03.2021 09:20

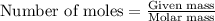
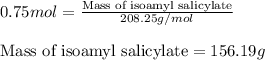
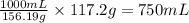 of solution.
of solution.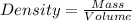
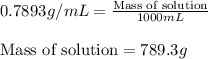
 of solution.
of solution.

