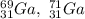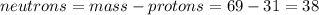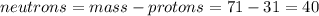Chemistry, 08.10.2020 14:01 norahfrost
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu.
Part A. How many protons and neutrons are in the nucleus of isotope with mass of 68.926 amu? Express your answers as an integers. Enter your answers numerically separated by a comma. p, n = .
Part B. How many protons and neutrons are in the nucleus of isotope with mass of 70.925 amu? Express your answers as an integers. Enter your answers numerically separated by a comma. p, n = .
Part C. Write the complete atomic symbol for each, showing the atomic number and mass number. Express your answers as isotopes separated by a comma.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2


Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 23.06.2019 08:30
Plz a person walks 1 mile every day for exercise, leaving her front porch at 9 am and returning to her front porch at 9: 25 am what was the total displacement of her daily walk a. 1 mile b. 0 c. 25 min d. none of the above
Answers: 2
You know the right answer?
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu.
Par...
Questions





Biology, 28.04.2021 21:30


Mathematics, 28.04.2021 21:30


Mathematics, 28.04.2021 21:30


Mathematics, 28.04.2021 21:30




Mathematics, 28.04.2021 21:30


History, 28.04.2021 21:30


Mathematics, 28.04.2021 21:30

Mathematics, 28.04.2021 21:30