Chemistry, 08.10.2020 14:01 justijust500
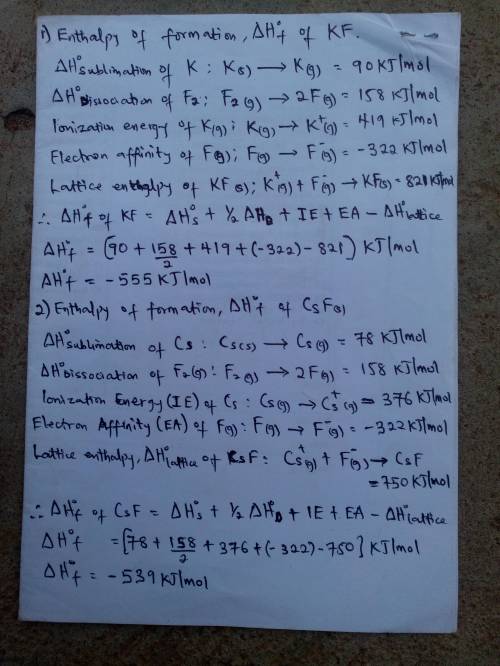
Calculate the enthalpies of formation, ΔHf∘, of the group 1 fluoride compounds from their elements using the Born–Haber cycle.
Process H∘, /
sublimation of K(s) 90
sublimation of Cs(s) 78
dissociation of F2(g) 158
ionization energy of K(g) 419
ionization energy of Cs(g) 376
electron affinity of F(g) −322
lattice enthalpy of KF(s) 821
lattice enthalpy of CsF(s) 750

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Calculate the enthalpies of formation, ΔHf∘, of the group 1 fluoride compounds from their elements u...
Questions

Mathematics, 24.05.2020 01:02

Law, 24.05.2020 01:02

History, 24.05.2020 01:02

Computers and Technology, 24.05.2020 01:02

English, 24.05.2020 01:02






Biology, 24.05.2020 01:02

Law, 24.05.2020 01:02



Law, 24.05.2020 01:02

History, 24.05.2020 01:02

French, 24.05.2020 01:02