Chemistry, 08.10.2020 07:01 musiclyhollywoodbabo
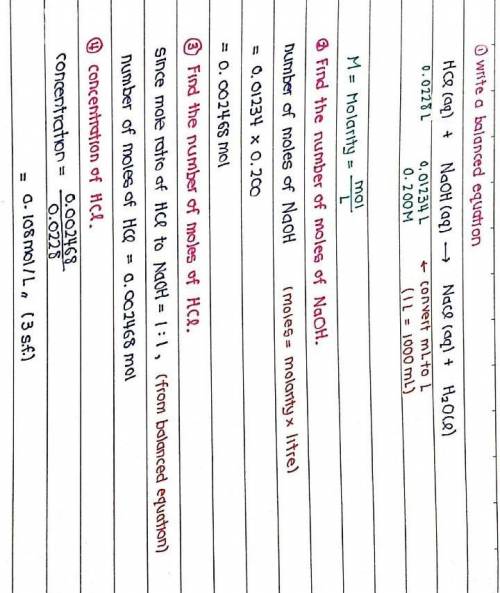
The titration of 22.80 mL of HCl solution of unknown concentration requires 12.34 mL of a 0.200 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution in M? Express your answer in moles per liter to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
The titration of 22.80 mL of HCl solution of unknown concentration requires 12.34 mL of a 0.200 M Na...
Questions

History, 06.05.2020 21:33







Chemistry, 06.05.2020 21:33


Mathematics, 06.05.2020 21:33






Social Studies, 06.05.2020 21:33

Mathematics, 06.05.2020 21:33