
How many σ and π bonds are in this molecule? A chain of five carbon atoms. There is a double bond between the first and second carbon atoms and a triple bond between the fourth the fifth carbon atoms. There are single bonds between the remaining carbon atoms. There are two hydrogen atoms bonded to the first carbon atom through single bonds, and a single hydrogen atom bonded to both the second and fifth carbon atoms through single bonds. There is an oxygen atom bonded to the third carbon atom through a double bond.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

You know the right answer?
How many σ and π bonds are in this molecule? A chain of five carbon atoms. There is a double bond be...
Questions









Computers and Technology, 12.11.2019 03:31











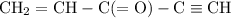 .
.


