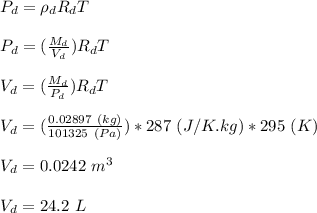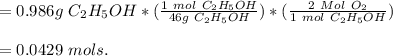Chemistry, 08.10.2020 03:01 diegomacias2411
A student wants to burn a 1.25 mL sample of ethanol (C2H5OH, d = 0.789 g/mL) in a jar containing dry air. Assuming the air in the jar is at standard atmospheric pressure and room temperature (22 °C), what volume will the jar need to be in order to hold enough oxygen for complete combustion? Hint: Refer to the composition of dry air in the previous question. (a) Write a balanced chemical reaction for the combustion of ethanol. (b) Calculate the moles of oxygen needed to completely combust the ethanol. (c) Calculate the partial pressure of oxygen in the jar. (d) Calculate the volume of oxygen (in L) needed in t

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
A student wants to burn a 1.25 mL sample of ethanol (C2H5OH, d = 0.789 g/mL) in a jar containing dry...
Questions




Chemistry, 16.09.2021 23:10

Mathematics, 16.09.2021 23:10





Mathematics, 16.09.2021 23:10

Mathematics, 16.09.2021 23:10






Mathematics, 16.09.2021 23:10

Mathematics, 16.09.2021 23:10


Mathematics, 16.09.2021 23:10