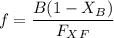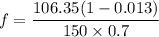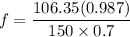Chemistry, 08.10.2020 01:01 joannakawata6
A distillation column is separating 150.0 kmol/h of a saturated liquid mixture that is 30.0 mol% methanol and 70.0 mol% water. The column operates at 1.0 atm pressure. Reflux ratio is 2.0, and reflux is returned as a saturated liquid. We desire a 97.0% recovery of methanol in the distillate and a methanol distillate mole fraction of 0.990. Find distillate ow rate D, bottoms flow rate B, methanol mole fraction in the bottoms xM, bot, and the fractional recovery of water in the bottoms.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

You know the right answer?
A distillation column is separating 150.0 kmol/h of a saturated liquid mixture that is 30.0 mol% met...
Questions

Mathematics, 04.02.2020 13:55



Mathematics, 04.02.2020 13:55

Biology, 04.02.2020 13:55



Mathematics, 04.02.2020 13:55


Mathematics, 04.02.2020 13:56

Biology, 04.02.2020 13:56




Mathematics, 04.02.2020 13:56

Biology, 04.02.2020 13:56




Mathematics, 04.02.2020 13:56

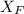 = 30%
= 30%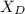 = 0.990
= 0.990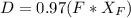
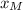 can be computed by using the formula:
can be computed by using the formula: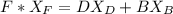
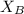 )
)