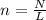Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2

You know the right answer?
How many moles are 2.50x10^21 atoms of silicon?...
Questions

Mathematics, 08.06.2021 22:10




Mathematics, 08.06.2021 22:10


English, 08.06.2021 22:10

Mathematics, 08.06.2021 22:10

Mathematics, 08.06.2021 22:10


Mathematics, 08.06.2021 22:10


Mathematics, 08.06.2021 22:10


Spanish, 08.06.2021 22:10

Mathematics, 08.06.2021 22:10



Mathematics, 08.06.2021 22:10

Mathematics, 08.06.2021 22:10