
Chemistry, 07.10.2020 14:01 VanBrocklin8501
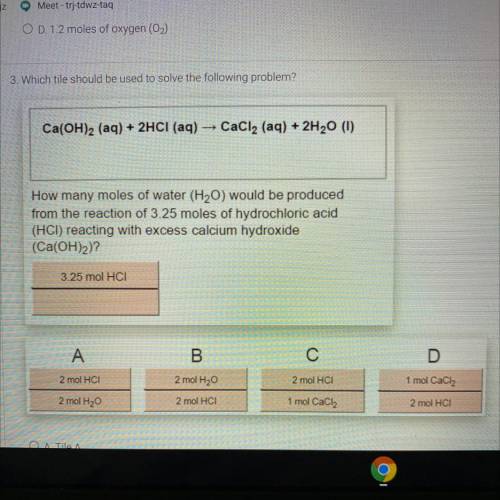
3. Which tile should be used to solve the following problem?
Ca(OH)2 (aq) + 2HCl (aq) → CaCl2 (aq) + 2H20 (1)
How many moles of water (H2O) would be produced
from the reaction of 3.25 moles of hydrochloric acid
(HCI) reacting with excess calcium hydroxide
(Ca(OH)2)?
3.25 mol HCI
A
B
С
D
2 mol HCI
2 mol H20
2 mol HCI
1 mol CaCl2
2 mol H20
2 mol HCI
1 mol CaCl2
2 mol HCI

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
3. Which tile should be used to solve the following problem?
Ca(OH)2 (aq) + 2HCl (aq) → CaCl2 (aq)...
Questions

History, 09.12.2021 03:50




History, 09.12.2021 03:50







SAT, 09.12.2021 03:50



Computers and Technology, 09.12.2021 03:50





Mathematics, 09.12.2021 03:50



