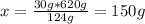Chemistry, 21.09.2019 01:30 Jalenmiller492
2ca3(po4)2 + 6sio2 + 10c --> p4 + 6casio3 + 10co how many grams of calcium phosphate are required to give 30.0g of phosphorus? ? i got 542g but i think that is way off. i did 30g p/30.97g to get .989 moles p • 2ca= 1.94 mole ca• 279.21g to get from miles to grams. explain how to do it and if you can where i went wrong.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1


Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3
You know the right answer?
2ca3(po4)2 + 6sio2 + 10c --> p4 + 6casio3 + 10co how many grams of calcium phosphate are require...
Questions