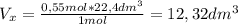Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 13:00
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2
You know the right answer?
Use the equation below to determine what volume of nitrous oxide (n20) can be produced from the deco...
Questions


History, 08.12.2020 17:20

Chemistry, 08.12.2020 17:20

English, 08.12.2020 17:20


Mathematics, 08.12.2020 17:20



History, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20

History, 08.12.2020 17:20





Health, 08.12.2020 17:20


English, 08.12.2020 17:20

Computers and Technology, 08.12.2020 17:20