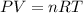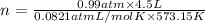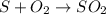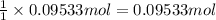Chemistry, 14.10.2019 21:40 shardonnay2160
What mass of sulfur has to burn to produce 4.5l so2 at 300°c and 101 kpa in the following reaction?
a. 13.5 g s
b. 3.07 g s
c. 68.8 g s
d. 41.0 g s

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2


Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
What mass of sulfur has to burn to produce 4.5l so2 at 300°c and 101 kpa in the following reaction?...
Questions


Mathematics, 10.05.2021 04:10


Biology, 10.05.2021 04:10


Mathematics, 10.05.2021 04:10

Social Studies, 10.05.2021 04:10


Mathematics, 10.05.2021 04:10




Mathematics, 10.05.2021 04:10

Mathematics, 10.05.2021 04:10


English, 10.05.2021 04:10

Business, 10.05.2021 04:10



Mathematics, 10.05.2021 04:20