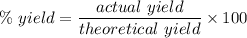Chemistry, 02.10.2020 21:01 briannahernand2
Compound A reacts with Compound B to form only one product, Compound C, and it's known the usual percent yield of C in this reaction is 40%. Suppose 10.0 g of A are reacted with excess Compound B, and 6.4 g of Compound C are successfully isolated at the end of the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

You know the right answer?
Compound A reacts with Compound B to form only one product, Compound C, and it's known the usual per...
Questions





Physics, 28.09.2020 15:01



Mathematics, 28.09.2020 16:01


History, 28.09.2020 16:01

Mathematics, 28.09.2020 16:01

English, 28.09.2020 16:01

Social Studies, 28.09.2020 16:01

Mathematics, 28.09.2020 16:01

Mathematics, 28.09.2020 16:01

Mathematics, 28.09.2020 16:01

Law, 28.09.2020 16:01

SAT, 28.09.2020 16:01


Mathematics, 28.09.2020 16:01