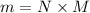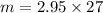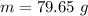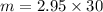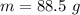Chemistry, 02.10.2020 17:01 trintrin227
Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia reacts with O2 to give nitric oxide, NO.
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Then nitric oxide reacts with methane, CH4.
2NO(g) + 2CH4(g) → 2HCN(g) + 2H2O(g) + H2(g)
When 50.2 g of ammonia and 48.4 g of methane are used, how many grams of hydrogen cyanide can be produced? How many grams of which reactant remain at the end of both reactions? (You may assume that O2 is in excess in the first reaction.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia reacts with O2 to give nitr...
Questions

English, 01.09.2019 20:20

Computers and Technology, 01.09.2019 20:20




Geography, 01.09.2019 20:20

Mathematics, 01.09.2019 20:20





Health, 01.09.2019 20:20



English, 01.09.2019 20:20


Mathematics, 01.09.2019 20:20

Biology, 01.09.2019 20:20

Mathematics, 01.09.2019 20:20

Social Studies, 01.09.2019 20:20

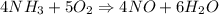
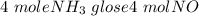
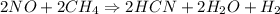
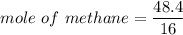
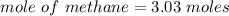
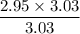 = 2.95 mol HCN
= 2.95 mol HCN